Explore the Right Options for the li-ion Battery
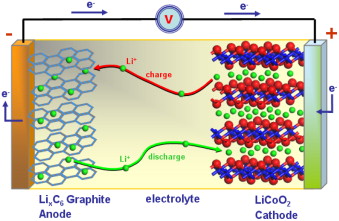
Electrolysis is widely used in industry, because it is possible to isolate some key substances for many production processes, such as aluminum, chlorine, sodium hydroxide, etc. In addition, it is also a process that purifies and protects coating of various metals. Electrolysis occurs only with power supply through a generator, such as a battery, for example. To understand for the graphite powder for li-ion battery anode this is the best deal here now.
To understand how it happens, look at the following scheme:
The generator pulls the electrons from the positive pole anode of the electrolyte bowl and transfers them to the negative pole (cathode). This is shown by the semi-reactions:
1st Half Reaction: The generator attracts the A- anions to the positive pole and forces them to lose electrons: THE- A0 + electron
2nd Semi-Reaction: The generator makes the C + cations receive the electrons: C ++ electron C0
There are two main types of electrolysis: igneous electrolysis and aqueous electrolysis. Understand the difference between them:
Igneous Electrolysis: Occurs when the passage of electric current occurs in a liquefied ionic substance, ie, fused. Hence the origin of the name “igneous”, a word that comes from Latin, igneous, means inflamed, ardent.
This type of reaction is widely used in industry, especially for the production of metals. See the example of NaCl (sodium chloride – salt) electrolysis, with a production of metallic sodium and chlorine gas:
Don’t stop now there’s more after the advertising:
- Cathode semi-reaction: Na + + and – → Na. (2)
- Anode semi-reaction: 2 Cl – → Cl 2 + 2e –
- Overall reaction: 2 Na + + 2 Cl – → 2 Na + Cl 2
Aqueous Electrolysis: In this case, the ions of the dissolved substance (solute) and water are included. In the electrolysis of sodium chloride in an aqueous medium, caustic soda (NaOH), hydrogen gas (H 2) and chlorine gas (Cl 2) are produced. Notice how it goes:
- NaCl Dissociation: 2 NaCl- → 2 Na + + 2 Cl –
- Water autoionization: 2 H2O → 2 H + + 2 OH –
- Cathode semi-reaction: 2 H + + 2e – → H 2
- Anode semi-reaction: 2 Cl – → Cl 2 + 2e –
- Overall reaction: 2 NaCl- + 2 H2O → 2 Na ++ 2 OH- + H2 + Cl2
Cathode anode solution
Note that two cations (Na + and H +) and two anions (Cl – and OH -) were formed. However, only one cation (H +) and one anion (Cl -) suffered electrode discharges, the other ions were only spectators in this electrolysis.
This occurs in all electrolysis in an aqueous medium: only one of the cations and one of the anions are participants. To determine which participants will be and which viewers will be, there is an order of ease of downloading as shown in the list:
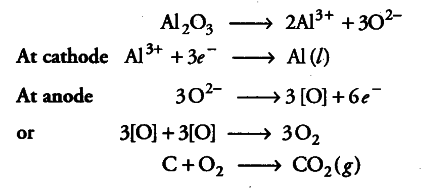
Thus, looking at the list, we see that H + cation are easier to discharge than Na + which is an alkali metal. And with respect to anions, Cl – is an unoxygenated anion and more reactive than OH-. The options are perfect in this case and therefore, you can go for the best at the moment.
